From Neural Induction to Brain MakingThe Scientific Legacy of Yoshiki Sasai
Table of Contents
- From Clinical Practice to Basic Research
- Identification of the Molecular Basis of the Spemann Organizer
- Identification of Downstream Factors Critical for Neural Differentiation
- Approaching the Development of a More Complex Brain
- Highly Reproducible Developmental Processes
- Application of Developmental Neurobiology to ES Cells
- Establishment of the SDIA Method for Inducing the Central Nervous System from ES Cells
- Induction of Retinal Pigment Epithelial Cells and Peripheral Nervous System Neurons from ES Cells
- Development of the SFEB Method for Inducing Cerebral Neural Tissue
- Applications of the SFEB Method: Induction of Photoreceptors, Cerebellar Purkinje Cells, and Hypothalamic Neurons
- From Cells to Tissues: Successful Self-organization of the Cerebral Cortex from ES Cells
- Self-organization of Three-dimensional Retinal Tissue
- Three-dimensional Formation of Pituitary Tissue
- The Brain Maker
- Contributions of Human ES Cells to Basic Research
- Integration of Developmental Biology, ES Cell Research, and Regenerative Medicine
- Awards
- Selected Publications
From Clinical Practice to Basic Research
After obtaining his MD from the Faculty of Medicine at Kyoto University in 1986, Dr. Sasai began his career as a medical intern in internal medicine. However, upon realising that the biological basis of many diseases remained unexplained, he decided to pursue research in neurobiology at the laboratory of Professor Shigetada Nakanishi at Kyoto University. In this laboratory, he identified Hes-1 and Hes-3 in vertebrates, transcription factors that play critical roles not only in neural development but also in a wide range of developmental processes[1].
Identification of the Molecular Basis of the Spemann Organizer
Upon completing his doctorate at Kyoto University, Dr. Sasai joined the laboratory of Eddy De Robertis at UCLA for his postdoctoral training, where the research focused on early development in Xenopus laevis. Research by Spemann and colleagues using amphibians had already demonstrated that the organizer possesses inductive activity toward the dorsal region, including neural tissue — a discovery that earned Spemann the Nobel Prize in 1935. However, the molecular identity of the organizer remained elusive at that time.
In the early 1990s, rapid advancements in molecular biology began to merge with developmental biology. Employing molecular biological techniques he had acquired at Kyoto University, Dr. Sasai aimed to uncover the molecular nature of the organizer and successfully identified Chordin[2]. The following year, he demonstrated that Chordin possesses neural-inducing activity. He found that overexpression of Chordin in animal caps — undifferentiated tissues derived from ectoderm that usually develop into epidermis but retain the potential to differentiate into mesoderm and endoderm — led to neural differentiation[3]. Furthermore, he showed that BMP4 antagonises the neural-inducing activity of Chordin. Later, it was revealed that the reciprocal inhibition between Chordin and BMP4 plays a central role not only in neural induction but also in the determination of dorsoventral axis[4]. Today, the Chordin-BMP4 system is recognized as being highly conserved across species, from fruit flies to vertebrates.

Identification of Downstream Factors Critical for Neural Differentiation
After returning to Kyoto University in 1996 as an associate professor and later a full professor — and from 2000 as a group director at the RIKEN Center for Developmental Biology (CDB) — Dr. Sasai continued his research on neural development. He analyzed the transcription factors induced during Chordin-mediated neuralization of animal caps and identified Zic-r1 and Sox2[5]. These transcription factors were suppressed when BMP4 inhibited neuralization. Overexpression of Zic-r1 was shown to induce neural tissue, while Sox2, when co-overexpressed with Fgf, also induced neural tissue. Additionally, SoxD was exclusively expressed in neuralized animal caps, and a dominant-negative form of SoxD suppressed Chordin-mediated neuralization[6]. These findings revealed that multiple transcription factors act cooperatively to regulate neural differentiation.
Dr. Sasai and colleagues also successfully identified XFDL156, a factor critical for ectodermal differentiation into neural tissue[7]. XFDL156 is specifically expressed in the ectoderm; its overexpression suppresses mesodermal differentiation, while inhibition of XFDL156 leads to excessive mesodermal differentiation. They further discovered that XFDL156 directly binds to p53, a mesoderm-inducing factor, and inhibits its function.
Approaching the Development of a More Complex Brain
In the early stages of development, neural tissue is induced by factors secreted from the organizer, such as Chordin. As development progresses, the neural tissue becomes subdivided into distinct regions, forming the complex structures of the brain. To understand brain development, it is essential to clarify how a simple neural tissue develops into the mature brain.
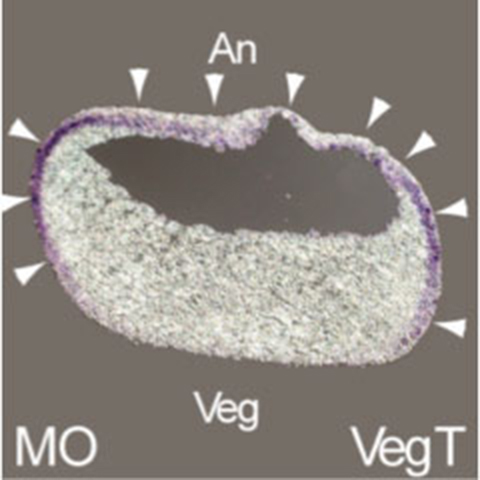
Dr. Sasai analyzed the genes involved in the regionalization of neural tissue and identified several key factors. For example, Tiarin, a secreted protein expressed in the anterior neural plate[8]. later shows restricted expression in the dorsal neural tube during development. Tiarin is essential for dorsal neural tube formation, as demonstrated by the dorsalization of neuralized animal caps. Similarly, Jiraiya was also reported to play a role in dorsalization[9]. This process depends on BMP signalling, with Jiraiya binding directly to BMPRII (BMP receptor II) and inhibiting the receptor's transport to the cell surface. Consequently, both overexpression and inhibition of Jiraiya affect dorsalization.
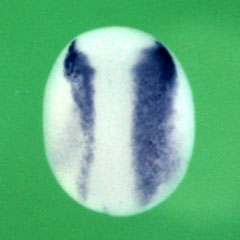
Dr. Sasai's group also identified genes regulating the anterior–posterior axis of neural tissue. For instance, they discovered that overexpression of XsalF promotes the anteriorization of neural tissue[10]. Anterior-posterior patterning is primarily regulated by Wnt signalling, where lower Wnt activity leads to anteriorization. XsalF positively regulates the expression of Wnt-inhibitory factors, thereby promoting anteriorization by suppressing Wnt signalling. Similarly, overexpression of Del1 induced anteriorization[11,12]. Del1, a secreted protein, suppresses Wnt signalling through the Ror2 pathway, demonstrating that high levels of Del1 expression promote anteriorization via Wnt inhibition.

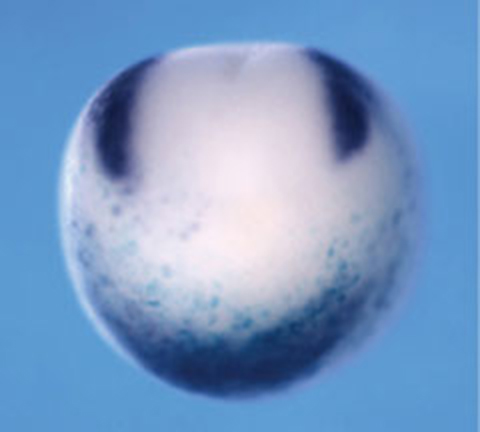
Furthermore, Dr. Sasai identified genes involved in the development of the peripheral nervous system. FoxD3 was shown to play a crucial role in the differentiation of neural crest cells into peripheral neurons[13]. Overexpression of FoxD3 in undifferentiated animal caps led to neural crest differentiation. Additionally, Pax3 and Zic1 were shown to act cooperatively in neural crest development[14]. Using Xenopus animal caps, which exclude influences from mesoderm and endoderm, Dr. Sasai elucidated the processes shaping the complex patterning of the head region.
Highly Reproducible Developmental Processes
In developmental processes, the formation of complex tissues, such as the brain, generally proceeds with remarkable accuracy. To ensure this high reproducibility, the developmental systems must possess robustness, allowing them to adapt appropriately to perturbations.
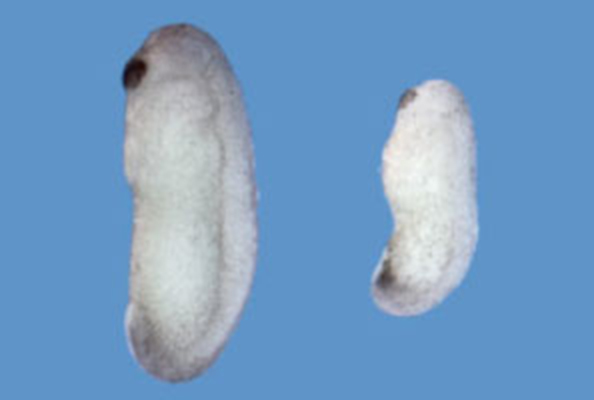
The formation of the dorsoventral axis in *Xenopus* represents one of the earliest patterning events, critically influencing subsequent development. This process is known to exhibit robustness against various disturbances. For example, slight changes in the amount of Chordin do not significantly affect dorsoventral patterning. However, the underlying mechanisms had long remained unclear. Dr. Sasai's group demonstrated that a molecule called ONT1 regulates Chordin levels by promoting its degradation when Chordin is excessive, and inhibiting its degradation when Chordin is insufficient, thereby maintaining a stable concentration[15]. They also showed that dorsoventral patterning scales according to embryo size. When a Xenopus embryo is bisected dorsally, the dorsal half can still develop into a tadpole of half the normal size while preserving proportional shape. This size-dependent robustness (scaling) was found to be mediated by Sizzled, which regulates Chordin stability in proportion to embryo size[16].
Application of Developmental Neurobiology to ES Cells
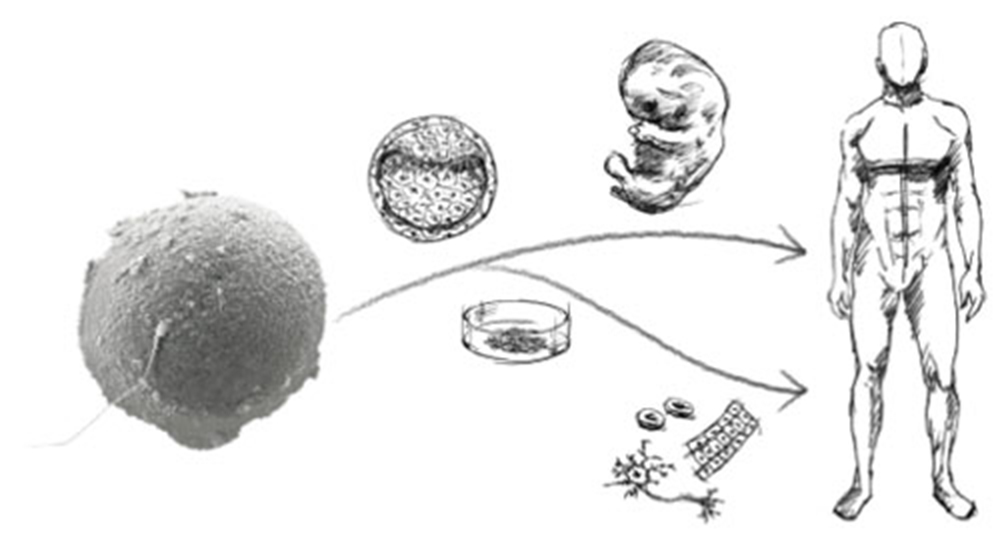
As described above, Dr. Sasai primarily used Xenopus laevis to elucidate the regulatory mechanisms of neural induction and head formation in vertebrates. He also succeeded in establishing methods to induce various neural tissues in vitro from pluripotent animal caps. Beginning around 1998, he energetically applied these approaches to mammals, aiming to induce neural tissues from embryonic stem (ES) cells, which retain the highest level of pluripotency.
While ES cells are often highlighted for their potential in regenerative medicine, Dr. Sasai strongly recognised their value for studying developmental biology. In mammals, where development takes place within the uterus, it is difficult to analyze the mechanisms of neural development and brain formation due to the complex tissue-tissue interactions. By inducing neural tissues from ES cells in vitro, however, it became possible to explore the mechanisms of brain formation logically and quantitatively, under conditions free from surrounding signals and tissue interactions. This laid the foundation for a new field, now known as “synthetic embryology,” which Dr. Sasai pioneered and through which he established global leadership.
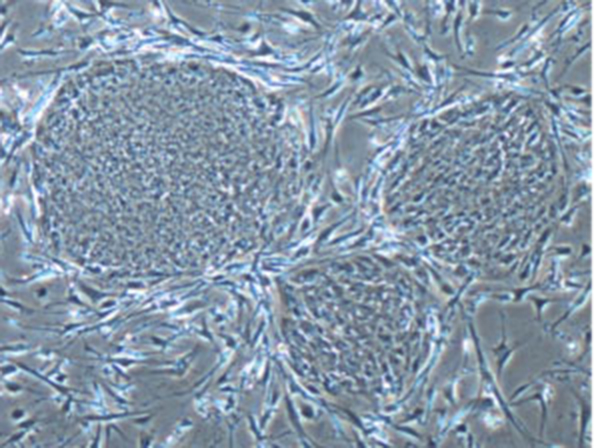
Establishment of the SDIA Method for Inducing the Central Nervous System from ES Cells
Around 2000, when Dr. Sasai moved from Kyoto University to RIKEN CDB, he published a landmark study that paved the way for a series of groundbreaking discoveries. In this work, he and his colleagues demonstrated that co-culturing mouse ES cells with feeder cells — such as those derived from bone marrow — could specifically induce central nervous system neurons. They named this neural-inducing activity SDIA (Stromal cell-Derived Inducing Activity)[17]. Notably, this approach was particularly effective at producing dopaminergic neurons, which, after being transplanted into the mouse brain, successfully integrated into the striatum while maintaining their characteristics. This significant achievement subsequently led to ongoing research at Kyoto University aimed at developing treatments for Parkinson's disease, a disorder caused by the degeneration of dopaminergic neurons.
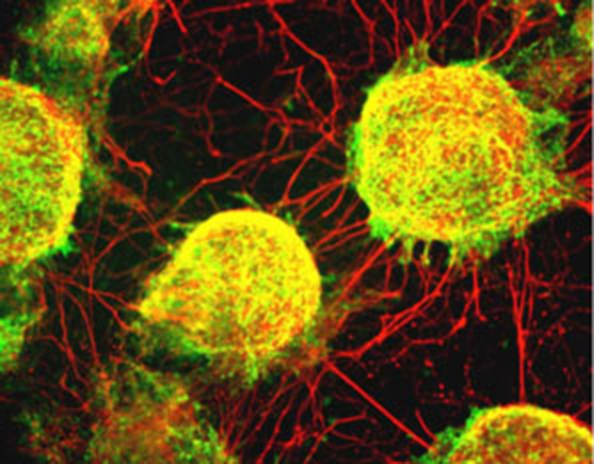
Induction of Retinal Pigment Epithelial Cells and Peripheral Nervous System Neurons from ES Cells
In 2002, Dr. Sasai's group further demonstrated that the SDIA method could also induce central nervous system neurons, including dopaminergic neurons, from primate ES cells[18]. Remarkably, they also discovered that retinal pigment epithelial (RPE) cells, which are derived from the central nervous system, could be induced using the SDIA method.
The protocol for generating RPE cells has since refined over more than two decades of research and has advanced to clinical studies led by Dr. Masayo Takahashi, CEO of Vision Care Inc., aimed at treating age-related macular degeneration (AMD). AMD is a condition in which RPE cells are damaged, resulting in vision loss. These clinical studies focus on develop fundamental therapies by producing RPE sheets from patient-derived induced pluripotent stem (iPS) cells and transplanting them beneath the retina.
Dr. Sasai's group continued to advance the SDIA method by fine-tuning culture conditions — specifically the timing, concentration, and combination of exogeneous signalling factors such as BMP4 and Shh. Through this approach, they demonstrated the selective induction of a broader range of neurons derived from both the central and peripheral nervous systems from ES cells[19].
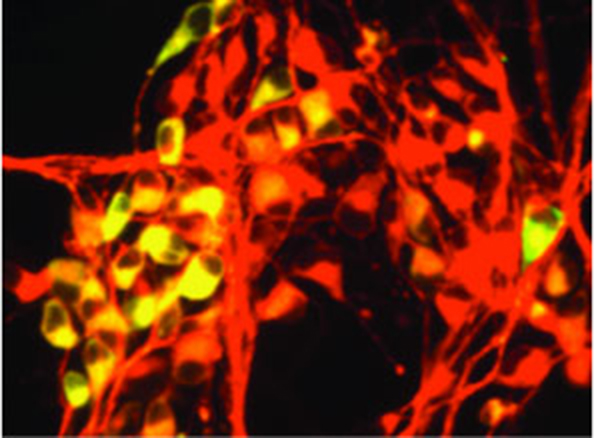
Development of the SFEB Method for Inducing Cerebral Neural Tissue
Existing methods, including SDIA, had shown limited ability to induce cerebral (telencephalic) neurons. To address this, Dr. Sasai and his team developed a novel culture method, culminating in the 2005 publication of the SFEB method (Serum-free Floating culture of Embryoid Body-like aggregates)[20].
In this approach, ES cells were cultured as "floating aggregates" without the need for co-culture with other cell types. The method efficiently induced neural cells, yielding a high frequency of telencephalic progenitors. Furthermore, by introducing signalling factors such as Shh and Wnt3a, the group demonstrated the selective induction of distinct telencephalic regions, including the cerebral cortex and basal ganglia, thereby achieving subregional specification within the telencephalon.
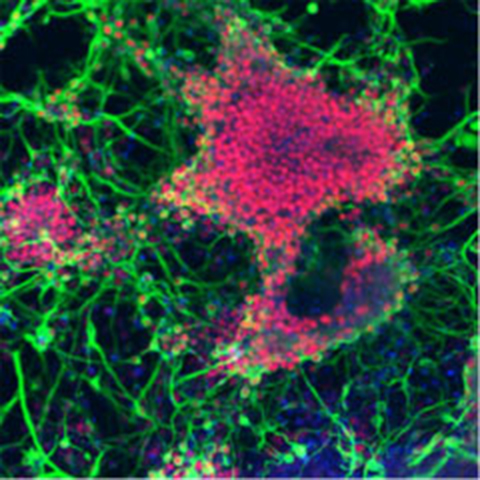
Applications of the SFEB Method: Induction of Photoreceptors, Cerebellar Purkinje Cells, and Hypothalamic Neurons
Building on the SFEB method, Dr. Sasai's group developed modified methods, including SFEB/DLFA, which enabled the induction of retinal photoreceptors through a stepwise process involving neural retinal progenitors[21]. While the induction of retinal pigment epithelial cells had already been achieved, the successful generation of photoreceptors — cells critical for vision — represented a major milestone.
They also established the SFEBq method, in which ES cells were rapidly reaggregated during floating culture. By sequentially adding multiple inducing factors, they succeeded in producing cerebellar Purkinje cells[22]. In brain development, the cerebellum is known to be induced by signals from the adjacent isthmic organizer. Remarkably, their culture system mimicked this process: ES cells initially gave rise to isthmic organizer-like tissue, which subsequently supported the differentiation of cerebellar cells. Thus, this approach succeeded in recapitulating even complex developmental processes that require inter-tissue interactions.
In a further conceptual breakthrough, the group demonstrated the induction of hypothalamic neurons by culturing ES cells under conditions devoid of insulin and other exogenous growth or inducing factors — long believed to be essential for cell survival[23]. This finding implied that the "default state" of central nervous system development includes the forebrain with the hypothalamus. Achieving these inductions required extensive trial and error in optimizing culture conditions, ultimately providing novel insights into the mechanisms of brain development.
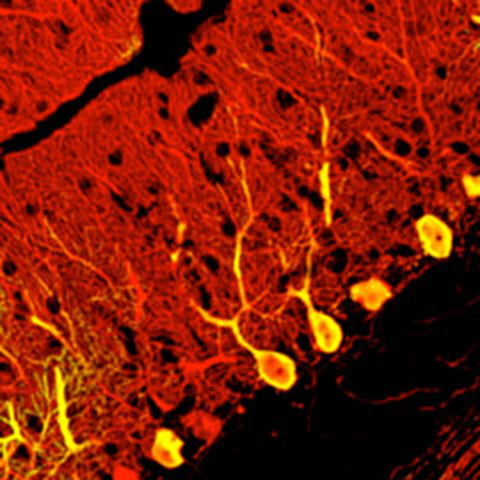
From Cells to Tissues: Successful Self-organization of the Cerebral Cortex from ES Cells
In 2008, Dr. Sasai's group made a major breakthrough that elevated ES cell differentiation research to a new level. By adapting the SFEB method, they successfully induced the formation of three-dimensional cerebral cortical tissue with layered structures from both mouse and human ES cells[24].
Until then, generating such complex, three-dimensional, in vivo-like tissue in vitro had been considered nearly impossible. However, using the SFEBq method for rapid aggregation, they efficiently obtained cortical progenitors, which, upon further culture, spontaneously developed into cortical tissue with a characteristic four-layer structure.
The discovery that ES cells alone — without interaction from surrounding tissues — could self-organize into such complex brain tissue astonished researchers worldwide.
By 2013, the group had established long-term culture methods for human ES cell-derived cortical tissue, enabling the observation of its maturation into structures corresponding to the human neocortex of the second trimester[25]. This achievement made it possible to observe human brain development in real time — an endeavor previously unattainable without ES or iPS cells.
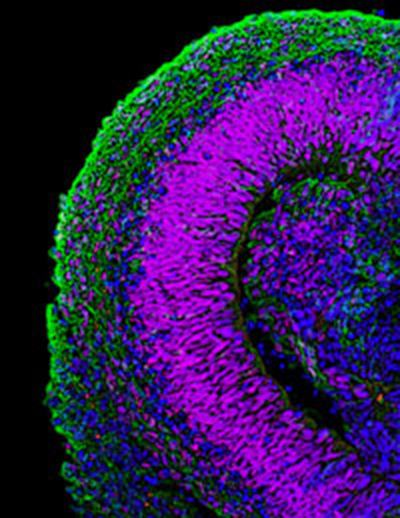
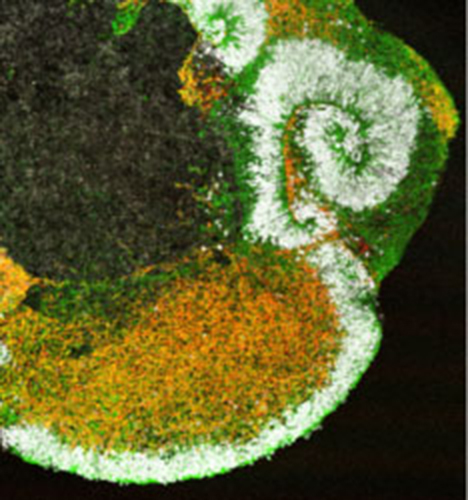
Self-organization of Three-dimensional Retinal Tissue
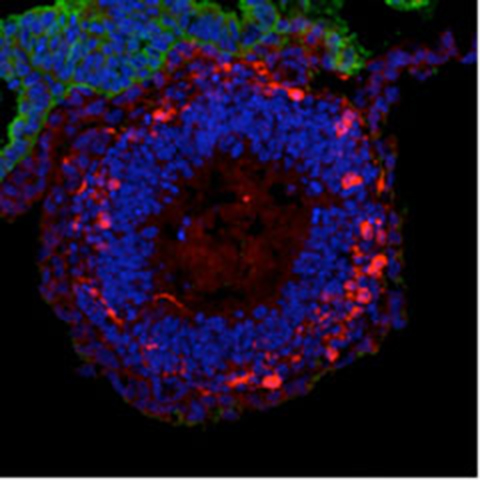
In 2011, Dr. Sasai and his colleagues achieved the formation of three-dimensional retinal tissue from mouse ES cells, marking a major advance in the study of self-organization[26].
Although previous studies had succeeded in inducing individual retinal components such as pigment epithelial cells and photoreceptors, this was the first instance in which an entire retinal tissue with a complex layered structure was generated in vitro. By introducing specific modifications to the SFEBq method, they directed ES cells to form neuroepithelium, which subsequently developed into optic vesicles and eventually optic cups — the developmental precursors of retinal tissue. Prolonged culture of the optic cups led to the formation of neural retina with multilayered structures resembling those of postnatal retinas.
Traditionally, developmental biology posited that optic cup formation required physical interactions with neighbouring tissues such as the lens and cornea. However, these ES cell experiments demonstrated that optic cup morphology could arise through intrinsic programmes alone. This finding opened new avenues for investigating the mechanisms underlying autonomous three-dimensional morphogenesis. In parallel, Dr. Sasai began pioneering biomechanical studies of tissue morphogenesis in this context.
His group subsequently succeeded in generating three-dimensional retinal tissues, including neural retina, from human ES cells[27]. Ophthalmology is considered one of the most promising fields in regenerative medicine; however, the intricate laminar structure of the neural retina presents major challenges for integrating transplanted cells. By enabling the generation of structured retinal tissue from pluripotent stem cells, this research greatly expanded the prospects of regenerative medicine for eye diseases.
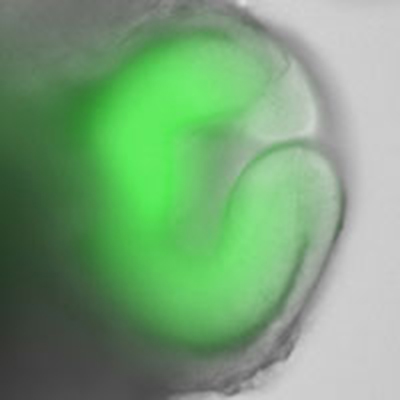
Three-dimensional Formation of Pituitary Tissue
Through modifications of the SFEBq method, Dr. Sasai's group also succeeded in inducing the self-organization of pituitary tissue from mouse ES cells[28].
A key factor in this success was the number of cells used at the onset of culture. By increasing the initial count to approximately 10,000 ES cells, the signalling environment within the aggregates was altered, enabling the simultaneous induction of hypothalamic cells and oral ectodermal cells. Interactions between these cell types led to the formation of Rathke's pouch, the primordium of the pituitary gland. Upon the addition of specific signalling factors, the tissue gave rise to various hormone-producing cells characteristic of the adenohypophysis, including ACTH-producing cells.
Transplantation of the ES cell-derived pituitary tissue into hypopituitary mice resulted in elevated serum ACTH levels and improvements in activity and survival. Given that the pituitary gland is the central regulator of hormone secretion throughout the body, this partial recapitulation of its development represented a major milestone.
The Brain Maker
Through these achievements, Dr. Sasai and his group succeeded in inducing not only diverse neural cell types but also three-dimensional neural tissues from ES cells. In recognition, a 2012 article in Nature referred to him as "THE BRAINMAKER."
In this interview, Dr. Sasai explained that rather than "push too hard and perturb the system", his approach was to "minimize external cues", allowing ES cells to follow their intrinsic developmental programmes for differentiation and self-organization. He remarked, "I don't want to be a parts-maker, making more and more tissues; I always want something conceptually different."
Perhaps his ultimate goal was to confront the question of how diverse neural tissues integrate into the brain, a highly complex organ — using ES cells as his investigative tool.
Contributions of Human ES Cells to Basic Research
Dr. Sasai and his colleagues also made significant contributions to the development of foundational technologies for human ES cells. One major achievement was elucidating the mechanism of cell death characteristic of human ES cells. When dissociated into single cells, human ES cells typically undergo rapid cell death, posing a major obstacle for gene transfer and large-scale culture.
His group discovered that this cell death was triggered by activation of Rho kinase and showed that inhibition of Rho kinase dramatically improved culture efficiency[29,30].
With an eye toward future applications in regenerative medicine, they also worked on developing culture methods entirely free of animal-derived components. For example, while the SDIA method for neural induction had previously required co-culture with animal cells, his team found that extracellular matrix components derived from human amniotic membrane exhibited similar inducing activity, enabling the induction of various neural cells from human ES cells in a completely animal-free environment[31].
They further revealed that, in the absence of extrinsic stimuli such as signalling factors, ES cells spontaneously differentiate into central nervous system tissue, particularly forebrain-like structures. This indicated that the "default state" of ES cell differentiation is toward the central nervous system — a phenomenon of great interest from both developmental and evolutionary perspectives.
In a 2011 study, they further identified the gene Zfp521 as a key trigger for this intrinsic neural differentiation of ES cells[32].
Integration of Developmental Biology, ES Cell Research, and Regenerative Medicine
As noted above, Dr. Sasai's group established culture conditions that enabled the induction of a wide variety of neural tissues from ES cells. Building on fundamental knowledge of developmental neurobiology, they carefully applied specific signalling factors in defined combinations and sequences. These advances have fed important insights back into the mechanisms of neural differentiation.
Thanks to the work of Dr. Sasai and other research groups, ES and iPS cells can now be induced to generate diverse neural tissues, greatly expanding the potential for applied research. The ability to observe living human neural tissues in vitro — a feat previously thought to be almost impossible — has had a profound impact on developmental neurobiology, disease modeling, and drug discovery. Furthermore, research aimed at using these cells for transplantation therapies in regenerative medicine has progressed rapidly.
Awards
Dr. Sasai received numerous prestigious awards in recognition of his groundbreaking contributions to developmental biology and stem cell research. These honors acknowledged his pioneering work in elucidating the molecular mechanisms of neural induction, as well as his success in generating diverse neural tissues and complex three-dimensional brain and retinal structures from ES cells. His achievements — bridging basic developmental biology and regenerative medicine — earned him a reputation as a global leader in the field.
- Human Frontier Science Program Organization 10th Anniversary Award (December 1998)
- Bälz Prize (November 2006; co-recipient)
- Commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology, Science and Technology Prize (April 2009)
- Tokyo Techno Forum 21 Gold Medal Award (April 2009)
- Osaka Science and Technology Award (October 2010)
- Inoue Prize for Science (February 2012)
- 6th Sayer Vision Research Lecture, Foundation of NIH (April 2012)
- Tsukahara Memorial Award (September 2012)
- Inoue Cultural Award (October 2012)
- Takeda Medical Science Prize (November 2012)
- Yamazaki-Teiichi Prize (November 2012)
- Systems Biology Pioneer Award (SPIE, USA) (May 2013)
- Hans Sigrist Prize 2013 (Hans Sigrist Foundation, University of Bern) (December 2013)
- Uehara Prize (March 2014)
Selected Publications
- Sasai, Y., Kageyama, R., Tagawa, Y.,Shigemoto, R. and Nakanishi, S. (1992) Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes and Dev. 12B, 2620-34
- Sasai Y., Lu, B., Steinbeisser H., Geissert D., Gont L. and De Robertis E. M. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specifichomeobox genes. Cell 79, 779-790
- Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E.M. (1995) Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336
- Piccolo, S., Sasai, Y., Lu, B., and De Robertis, E.M. (1996) Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell 86, 589-598
- Mizuseki, K., Kishi, M., Matsui, M., Nakanishi, S. and Sasai, Y. (1998) Xenopus Zic-related-1 and Sox-2, two factors inducer by Chordin, have distinct activities in the initiation of neural induction. Development 125, 579-587
- Mizuseki, K., Kishi, M., Shiota, K., Nakanishi, S. and Sasai, Y. (1998) Sox-D is an essential mediator for induction of anterior neural tissues in Xenopus embryos. Neuron 21, 77-85
- Sasai, N., Yakura, R,, Kamiya, D., Nakazawa, Y. and Sasai, Y. (2008) Ectodermal Factor Restricts Mesoderm Differentiation by Inhibiting p53. Cell 133, 878-890
- Tsuda,H., Sasai, N., Matsuo-Takasaki, M, Sakuragi, M., Murakami, Y. and Sasai, Y. (2002) Dorsalization of the Neural Tube by Xenopus Tiarin, a Novel Patterning factor Secreted by the Flanking Non-Neural Head Ectoderm. Neuron 33, 515-528
- Aramaki, T., Sasai, N., Yakura, R. and Sasai, Y. (2010) Jiraiya Attenuates BMP Signaling by Interfering with Type-II BMP Receptors in Neuroectodermal Patterning. Dev. Cell 19, 547-561
- Onai, T., Sasai, N., Matsui, M. and Sasai, Y. (2004) Xenopus XsalF: Anterior Neuroectodermal Specification by Attenuating Cellular Responsiveness to Wnt Signaling. Dev. Cell 7, 95-106
- Arakawa, A., Matsuo-Takasaki, M., Takai, A., Inomata, H., Matsumura, M., Ikeya, M., Takahashi, K., Miyachi, Y., Sasai, N. and Sasai,Y. (2007) The secreted EGF-Discoidin factor xDel1 is essential for dorsal development of the Xenopus embryo. Dev. Biol. 306, 160-169
- Takai, A., Inomata, H., Arakawa, A., Matsuo-Takasaki, M. and Sasai, Y. (2010) Anterior neural development requires Del1, a matrix-associated protein that attenuates canonical Wnt signaling via the Ror2 pathway. Development 137, 3293-3302
- Sasai, N., Mizuseki, K. and Sasai, Y. (2001) Requirement of FoxD3-class Signaling for Neural Crest Determination in Xenopus. Development 128, 2525-2536
- Sato, T., Sasai., N. and Sasai, Y. (2005) Neural Crest Determination by Co-activation of Pax3 and Zic1 Genes in Xenopus Ectoderm. Development 132, 2355-2363
- Inomata, H., Haraguchi, T. and Sasai, Y. (2008) Robust Stability of the Embryonic Dorsal Axial Pattern Requires ONT1, a Secreted Scaffold for Chordin Degradation. Cell 134, 854-865.
- Inomata H, Shibata T, Haraguchi T, Sasai Y. (2013) Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann's organizer signals. Cell 153, 1296-1311.
- Kawasaki, H., Mizuseki, K. , Nishikawa, S., Kaneko, S., Kuwana, Y., Nakanishi, S., Nishikawa, S.-I. and Sasai, Y. (2000) Induction of midbrain dopaminergic neurons from ES cells by Stromal Cell-Derived Inducing Activity. Neuron 28, 31-40.
- Kawasaki, H., Suemori, H., Mizuseki, K., Watanabe, K., Urano, F., Ichinose, H., Haruta, M., Takahashi, M., Yoshikawa, K., Nishikawa, S.i., Nakatsuji, N. and Sasai, Y. (2002) Generation of TH+ Dopaminergic Neurons and Pax6+ Pigment Epithelia from Primate ES cells by SDIA. Proc. Natl. Acad. Sci. USA 99, 1580-1585
- Mizuseki, K., Sakamoto, T., Watanabe, K., Muguruma, K., Ikeya, M., Nishiyama, A., Arakawa, A., Suemori, H., Nakatsuji, N., Kawasaki, H., Murakami, F. and Sasai, Y. (2003) Generation of Neural Crest-Derived PNS Neurons and Floor Plate Cells from Mouse and Primate ES Cells. Proc. Natl. Acad. Sci. USA 100, 5828-5833
- Watanabe, K., Kamiya, D., Nishiyama, A., Katayama, T., Nozaki, S., Kawasaki, H., Mizuseki, K., Watanabe, Y., and Sasai, Y. (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288-296
- Ikeda, H., Watanabe, K., Mizuseki, K., Haraguchi, T., Miyoshi, H., Kamiya, D., Honda, Y., Sasai, N., Yoshimura, N., Takahashi, M. and Sasai, Y. (2005) Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. USA 102, 11331-11336
- Muguruma, K., Nishiyama, A., Ono, Y., Miyawaki, H., Mizuhara, E., Hori, S., Kakizuka, A., Obata, K., Yanagawa, Y., Hirano, T. and Sasai, Y. (2010) Ontogeny-recapitulating generation and tissue integration of ES cell-derived cerebellar Purkinje cells. Nat. Neurosci. 13, 1171-1180
- Wataya, T., Ando, S., Muguruma, K., Ikeda, H., Watanabe, K., Eiraku, M., Kawada, M., Takahashi, J., Hashimoto, N. and Sasai, Y. (2008) Minimization of Exogenous Signals in ES Cell Culture Induces Rostral Hypothalamic Differentiation. Proc. Natl. Acd. Sci. USA 105, 11796-11801.
- Eiraku, M, Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura, S., Matsumura, M., Wataya, T., Nishiyama, A., Muguruma, K. and Sasai, Y. (2008) Self-Organized Formation of Polarized Cortical Tissues from ES cells and its Active Manipulation by Extrinsic Signals. Cell Stem Cell 3, 519-532
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M. and Sasai Y. (2013) Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA. 110, 20284-20289.
- Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. and Sasai, Y. (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56.
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M. and Sasai Y. (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771-785.
- Suga, H., Kadoshima, T., Minaguchi, M., Ohgushi, M., Soen, M.,Nakano, T., Takata, N., Wataya, T., Muguruma, K., Miyoshi, H., Yonemura, S., Oiso, Y. & Sasai, Y. (2011) Self-formation of functional adeno-hypophysis in three-demensional culture. Nature 480, 57-62
- Watanabe, K., Ueno, M., Kamiya, D., Nishiyama, A., Matsumura, M., Wataya, T., Takahashi, J.B., Nishikawa, S., Nishikawa, S.-i., Muguruma, K. and Sasai, Y. (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681-686
- Ohgushi, M., Matsumura, M., Eiraku, M., Murakami, K., Aramaki, T., Nishiyama, A., Muguruma, K., Nakano, T., Suga, H., Ueno, M., Ishizaki, T., Suemori, H., Narumiya, S., Niwa, H. and Sasai, Y. (2010) Molecular Pathway and Cell State Responsible for Dissociation-Induced Apoptosis in Human Embryonic Stem Cells. Cell Stem Cell 7, 225-239
- Ueno, M., Matsumura, M., Watanabe, K., Nakamura, T., Osakada, F., Takahashi, M., Kawasaki, H., Kinoshita, S., and Sasai, Y. (2006) Neural Conversion of Embryonic Stem Cells by an Inductive Activity on Human Amniotic Membrane Matrix. Proc. Natl. Acad. Sci. USA 103, 9554-9559
- Kamiya, D., Banno, S., Sasai, N., Watanabe, K., Kawada, M., Yakura, R., Jakt , L.M., Nishikawa, S. and Sasai. Y. (2011) Intrinsic transition of ES cell differentiation into neural progenitors. Nature 470, 503-509